The global life sciences industry, which includes pharmaceuticals, biotechnology, and medical devices, has been on the rise, growing more rapidly than ever over the past decade. One of the key drivers of this growth has been digital transformation, also referred to as “data-driven transformation.” But to sustain this growth over the next decade, the life sciences industry needs to make some fundamental changes in the way it approaches data, and a key change is that the industry must embrace cloud information management.
Data Disruptors & Data Silo Challenges
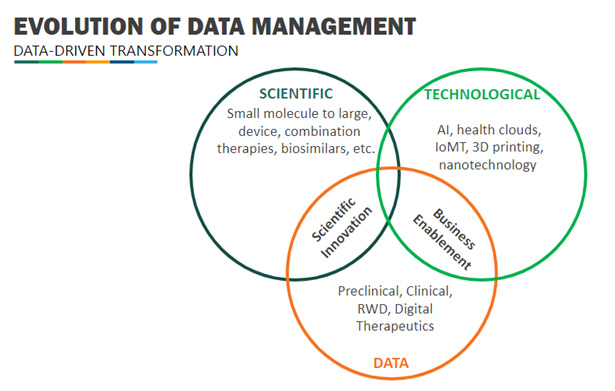
As we explore this data-driven transformation, there are three types of disruptors that must be identified:
- Scientific – small to large molecule, devices, combination therapies, bio similars, etc.
- Technological – Artificial Intelligence (AI), health clouds, Internet of Medical Things (IoMT), 3D printing, Nanotechnology, etc.
- Data – preclinical, clinical, real-world data (RWD), digital therapeutics, Data.gov, opensource, PubMed®, etc.
Most life sciences companies are running a “vertical or silo” operating model, with each silo using an enterprise application, such as CRM for Commercial, an ERP/MRP system for Supply Chain/Manufacturing, a Clinical Trial Management system (CTMS) for R&D, etc. Many of these systems “touching the product” become systems of record. These systems of record must comply with Good x Practice (GxP) requirements and can be audited any time by local health authorities and regulatory bodies.
Some of the data firewalls across departments are natural silos (e.g., Commercial and Medical) as these are necessary to stay compliant with regulations. Similarly, there is a separation based on validation, for example GxP vs. Non GxP systems. However, there are also organizational silos with no legal or regulatory barrier to information flow and innovation. Breaking down such silos is no easy task for any life sciences leader chartered to drive the data-driven transformation.
Given the volume and complexity of applications in the enterprise landscape, most life sciences organizations have neither not yet fully transitioned to cloud or are often running a hybrid multi-cloud platform environment. The life sciences disruptors, on the other hand, are often cloud native, do not have to deal with such technical deficiencies and can move nimbly and quickly in the marketplace.
Building An Enterprise Data Management (EDM) Strategy at the Leadership Level
Many life sciences companies have announced a Chief Data Officer (CDO) or expanded the role of Chief Information Officer (CIO) into a Chief Information and Digital Officer responsible for driving a data-driven digital transformation journey. These executives are often very savvy with data, the cloud, AI, design thinking, etc. and expect a level of cooperation between business and tech teams. These leaders are charged with changing the mindset of the enterprise into one that is agile, where most professionals view their job as focused on the unmet needs of patients and healthcare professionals (HCPs). Actionable insights are key to addressing these unmet needs.
Defining the data strategy and aligning everyone on the implementation roadmap is often a tough undertaking. Breaking the data silos to build an EDM approach where data is viewed as an asset, just like any other business asset, is a key principle to get right early in the transformation journey.
EDM Optimizes Emerging Clinical Study Models
As the life sciences industry embraces EDM and scales newer innovative ways of working, some of the trends that have emerged bring in newer data sources such as the ones involved in decentralized clinical trials (DCT), RWD, etc.
For example, DCTs have been a game-changer by incorporating mobile technology into clinical trials using smartphones, tablets, notebook computers, and wearable devices to allow for a clinical study to be designed accordingly and successfully run remotely.
With the right EDM and Enterprise Data Governance in place, DCTs help improve patient convenience and engagement, reduce clinical trial timelines, allow for more diverse patient populations, and reduce cost of conducting the trials.
Cloud-Based EDM a Catalyst for Life Sciences Clinical & Commercial Success
EDM is key to ensuring effective methods for conducting drug discovery, optimizing clinical trials, manufacturing, and optimizing supply & distribution and commercialization paths. Success stories with EDM have applied FAIR principles in addition to data as an asset; Findability, Accessibility, Interoperability, and Reuse of digital assets by ensuring the authenticity, integrity, and availability of data over its entire lifecycle.
EDM Strengthens Data Security & Compliance
With growing data privacy and cyber security concerns, EDM has become a boardroom topic, especially, as medicine gets more precise and personalized. We are observing EDM also being pursued by regulators, such as the Food and Drug Administration (FDA) in the US.
Data governance also plays a vital role in optimizing data accessibility while preventing data access from unauthorized users by enforcing data sharing decisions to be driven by safety and integrity.
Across the life science value chain, data compliance is an integral part of data management. To ensure regulatory compliance, the data platform required a robust validation process with a well-defined methodology to address US FDA, the European Medicines Agency (EMA), the Medicines and Healthcare products Regulatory Agency (MHRA), the Saudi Food and Drug Authority (SFDA), European Union (EU) directives, and other geo-specifics requirements.
EDM Platform Options & Axtria DataMAx™
As a response to these EDM challenges, Axtria has developed the Axtria DataMAx™ solution. Axtria DataMAx is a comprehensive, cloud-based data management platform, specifically designed for life sciences. It combines data onboarding and ingestion, processing, and transformation, stewardship, and data governance, to provide end-to-end data delivery along the entire data journey from onboarding new data sources, through ingestion, processing, and stewardship, to provisioning data for reporting, analysis, and other downstream consumption.
By leveraging DataMAx, Axtria’s clients can accelerate time to insight, improve data quality, and understand the journey that the data has undergone. This improved understanding thereby increases trust in the data, and trusted data enables informed, trusted insights.
Authors
 ANURAG CHATURVEDI Principal, Axtria Life Sciences Industry Practice |
 BHAGIRATH GOPINATH Principal, Axtria Business Information Management |
 NEIL STOKES Senior Director, Axtria Business Information Management |












