The human knowhow is well-known for being expansive beyond all limits, and yet there remains an awful little that we know better than growing on a consistent basis. This unwavering commitment towards growth, under every possible situation, has really brought the world some huge milestones, with technology emerging as quite a major member of the group. The reason why we hold technology in such a high regard is, by and large, predicated upon its skill-set, which guided us towards a reality that nobody could have ever imagined otherwise. Nevertheless, if we look beyond the surface for one hot second, it will become abundantly clear how the whole runner was also very much inspired from the way we applied those skills across a real world environment. The latter component, in fact, did a lot to give the creation a spectrum-wide presence, and as a result, initiated a full-blown tech revolution. Of course, the next thing this revolution did was to scale up the human experience through some outright unique avenues, but even after achieving a feat so notable, technology will somehow continue to bring forth the right goods. The same has turned more and more evident in recent times, and assuming one new discovery ends up with the desired impact, it will only put that trend on a higher pedestal moving forward.
The researching team at U.S. Department of Energy’s (DOE) Argonne National Laboratory has reportedly discovered an intriguing cooperative behavior that occurs among complex mixtures of components across electrolytes in batteries. To give you some idea, electrolytes are basically materials that move charge-carrying particles known as ions between a battery’s two electrodes, thus converting stored chemical energy into electricity. Anyway, they got to know how combining two different types of anions (negatively charged ions) with cations (positively charged ions) can significantly improve the overall battery’s performance. This has birthed a belief that careful selection of ion mixtures can enable battery developers to precisely tailor their devices to produce desired performance characteristics. To understand the importance carried by such a development, we must acknowledge that lithium-ion batteries used today actually have a limited ability to provide performance attributes needed in critical applications like passenger electric vehicles and storing renewable energy on the grid. Given these limitations, researchers across the globe have started to deem multivalent batteries as a potentially better alternative. Multivalent batteries, in essence, use cations such as zinc, magnesium, and calcium that have a charge of +2 as opposed to +1 for lithium ions. Boasting a greater charge stock, multivalent batteries are able to store and release more energy. Apart from it, the stated technology use abundant elements supplied through stable, domestic supply chains, something which looks a lot better when you consider lithium is less abundant and has an expensive, volatile international supply chain. Such a setup not only makes multivalent batteries a more viable alternative for electric vehicles, but they also have a use case around grid storage.
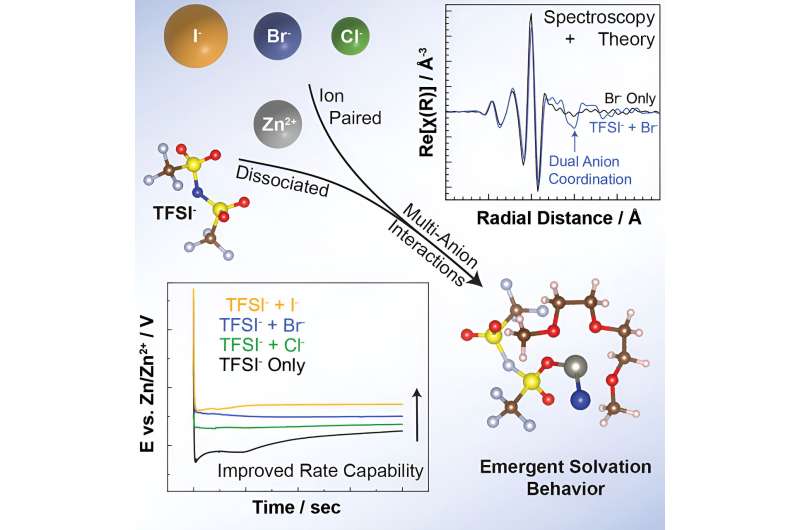
Having covered their advantages, we must also mention that most multivalent batteries taken under investigation by researchers have failed to perform well. This is because ions and electrodes tend to be unstable and degrade, turning it difficult for electrolytes to efficiently transport cations. The said problem eventually diminishes the battery’s ability to generate and store electricity. So, how does the new discovery helps the case of multivalent batteries? As zinc metal forms is among their main foundations, the researching team made an effort to characterize the interactions that occur— and the structures that form— when zinc cations are combined with two different types of anions in the electrolyte. This effort included designing a laboratory-scale battery system comprised of an electrolyte and zinc anode. The electrolyte initially contained zinc cations and an anion called TFSI. Here, they observed very weak attraction to the cations. Next up, they instilled chloride anions to the electrolyte. Going by the available details, chloride showed a much stronger attraction to zinc cations. However, that’s not where things ended. Researchers built upon their initial findings with three complementary techniques. In the first one, they used X-ray absorption spectroscopy, which was conducted at Argonne’s Advanced Photon Source, a DOE Office of Science user facility. This one involved probing the electrolyte using synchrotron X-ray beams for the purpose of measuring the absorption of the X-rays. Then, there was Raman spectroscopy technique. Conducted at Argonne’s Electrochemical Discovery Laboratory, the said technique sprung to action by illuminating the electrolyte with laser light before evaluating the scattered light. Lastly, they put into practice Density functional theory at Argonne’s Laboratory Computing Resource Center where the team stimulated and calculated the structures formed by the interactions among the ions in the electrolyte.
“These techniques characterize different aspects of the ion interactions and structures,” said Mali Balasubramanian, a physicist on the research team and one of the study’s authors. “X-ray absorption spectroscopy probes how atoms are arranged in materials at very small scales. Raman spectroscopy characterizes the vibrations of the ions, atoms and molecules. We can use the data on atom arrangements and vibrations to determine whether ions are separated or move together in pairs or clusters. Density functional theory can corroborate these characterizations through powerful computation.”
Owing to their extensive investigation, the researchers were able to figure out that the presence of chloride induced TFSI anions to pair with zinc cations. This marked a significant point, as the pairing of anions with a cation can affect the rate at which the cation can be deposited as metal on the anode during charging. Not just that, it can also have a similar impact when the cation is being stripped back into an electrolyte during discharge. For the sake of re-confirming their findings, the researchers repeated these experiments with two other ion mixtures. In the first ion mixture, they swapped chloride for bromide ions, whereas the other one saw them picking iodide ions over chloride. The results included bromide and iodide’s success in inducing TFSI anions to pair with zinc cations.
“What was particularly exciting about this result is that we didn’t expect to see what we saw. The idea that we can use one anion to draw a second anion closer to a cation was very surprising,” said Justin Connell, a materials scientist on the research team and one of the study’s authors.
Although the study seems pretty significant as a whole, one area where the team placed a special emphasis talked to the cooperation which occurred among different types of ions in an electrolyte. In simple terms, it meant the presence of the weakly attracting anions reduced the amount of energy needed to pull zinc metal out of solution. On the other hand, the presence of strongly attracting anions reduced the amount of energy needed to put the zinc back in solution. Such a coordination mandated less energy to facilitate a constant flow of electrons.
“Our observations highlight the value of exploring the use of different anion mixtures in batteries to fine-tune and customize their interactions with cations,” said Connell. “With more precise control of these interactions, battery developers can enhance cation transport, increase electrode stability and activity, and enable faster, more efficient electricity generation and storage. Ultimately, we want to learn how to select the optimal combinations of ions to maximize battery performance.”
For the future, the plan is to investigate the potential of other multivalent cations like magnesium and calcium interacting with various anion mixtures. Beyond that, the researchers will also dabble with machine learning to rapidly calculate the interactions, structures and electrochemical activity that occur in and around many different ion combinations. The latter approach, if found feasible, should accelerate the selection of most promising combinations.